Proton Dissociation and Transfer in PEM Ionomers with Multiple and Distinct Pendant Acid Groups: An Ab Initio Study
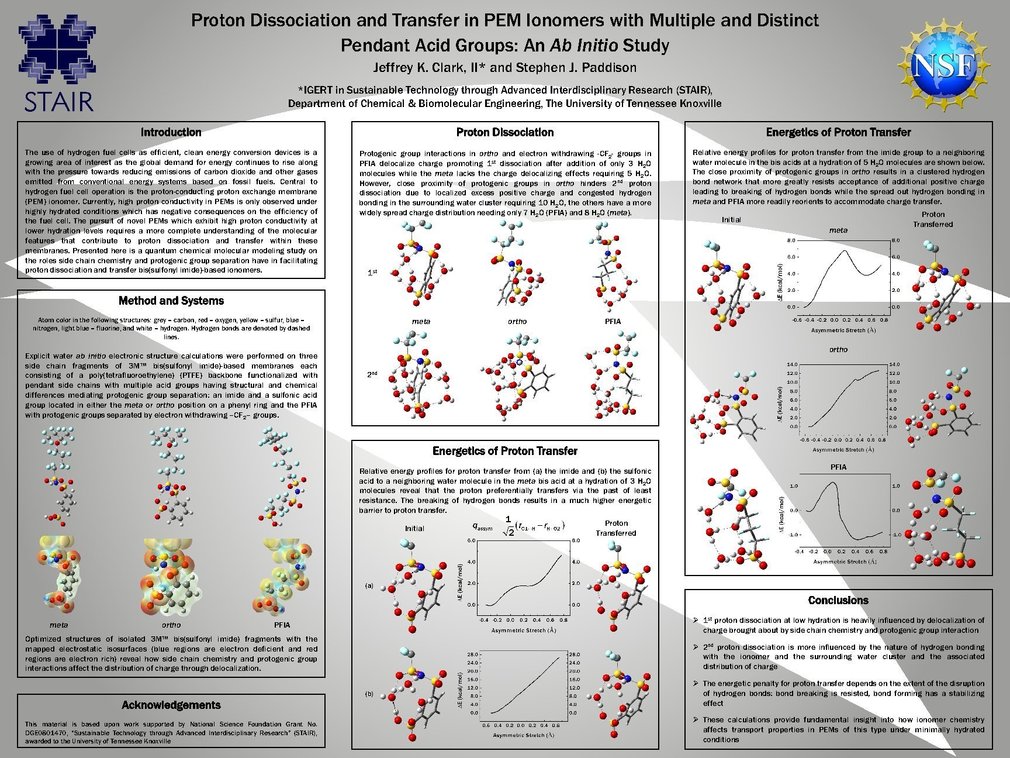
Proton Exchange Membrane (PEM) fuel cells offer tremendous potential as clean alternative energy conversion systems. Critical to these devices is the proton-conducting polymer electrolyte membrane. A molecular-level understanding of the factors that contribute to proton dissociation and transport in PEMs at low hydration levels is of vital importance in the development of novel proton conducting electrolyte materials for fuel cell application. Ab initio electronic structure calculations were performed to study the effects local hydration, protogenic group separation, and specific side chain chemistry have in facilitating proton dissociation and transfer in fragments of 3M bis(sulfonyl imide)-based PEM ionomers under conditions of low hydration. Three ionomers containing multiple acid groups per pendant side chain with structural and chemical differences mediating protogenic group separation were considered (side chains: –O(CF2)4SO2(NH)-SO2C6H4SO3H) with the sulfonic acid group located in either the meta or the ortho position on the phenyl ring and –O(CF2)4SO2(NH)SO23SO3H). Fully optimized structures of these fragments with the addition of water molecules revealed that both side chain chemistry and protogenic group separation are key contributors to proton dissociation and the energetics of proton transfer in these materials. Specifically, cooperative interaction between protogenic groups through hydrogen bonding and electron withdrawing –CF2– groups were found to be critical for first proton dissociation and the state of the dissociated proton at low levels of hydration. These factors, along with the surrounding hydrogen bond network, were also observed to have strong influence on the energetic penalty associated with proton transfer.



Judges and Presenters may log in to read queries and replies.