Adatom Emission from Nanoparticles: Implications for Ostwald Ripening
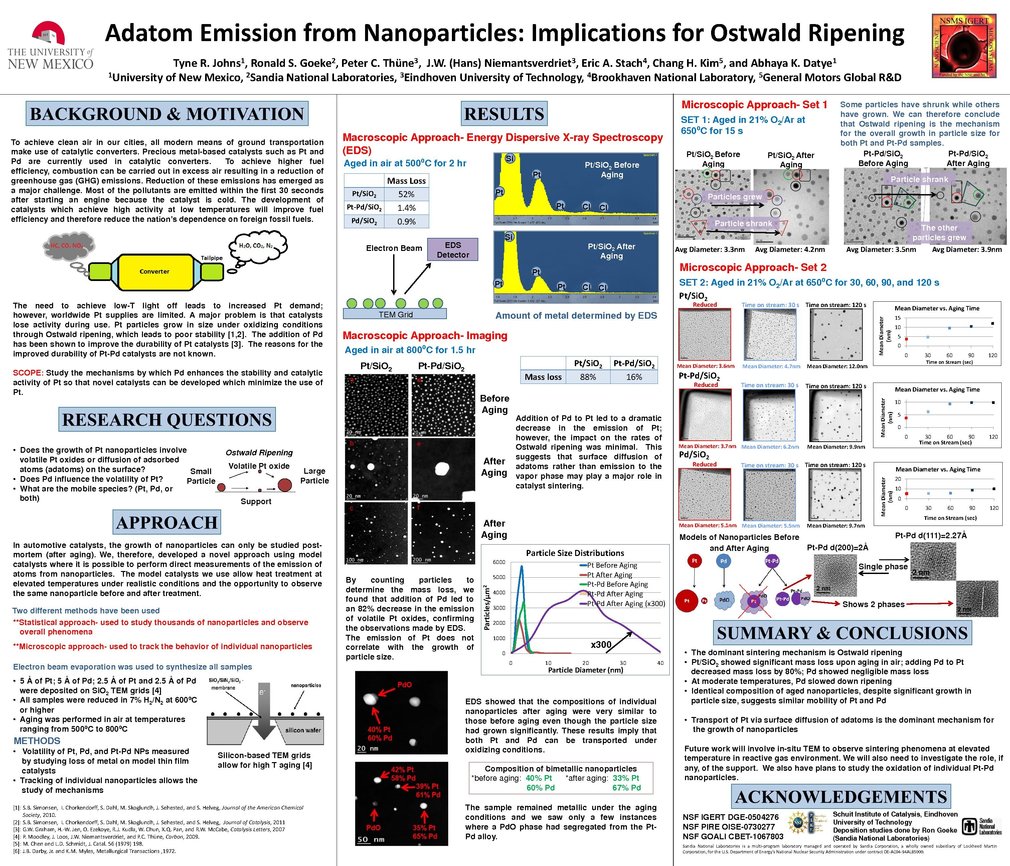
Catalysts are essential for the treatment of carbon monoxide, hydrocarbons, and nitrogen oxides in engine exhaust. Platinum (Pt) and palladium (Pd) are currently used in catalytic converters to transform these pollutants into less harmful molecules. The supply of precious metals is decreasing while demand for clean energy is increasing. Therefore, there is a need to develop more active catalysts with minimal use of precious metals such as Pt. A serious problem facing catalysts is the loss of activity during use. Several research groups have shown that Pt particles grow readily under oxidizing conditions, leading to poor durability, but it has been shown that addition of Pd to Pt leads to improved stability; however, it is unknown why.
It is suspected that volatile platinum oxides may be responsible for the rapid growth of Pt catalysts, but the relative importance of the surface and gas phase processes is not known. In automotive catalysts, this phenomenon can only be studied after aging. Therefore, we have developed a novel approach using model catalysts where it is possible to perform direct measurements of the emission of atoms from nanoparticles. Pt, Pd, and bimetallic Pt-Pd samples were created on TEM grids. The samples were reduced and aged under oxidizing conditions at different temperatures. We used a statistical approach and a microscopic approach to study the phenomena. Our results show that Pd impedes the emission of the volatile Pt oxides in the bimetallic samples; however, the vapor phase process does not fully explain the observed rates of Ostwald ripening.




Judges and Presenters may log in to read queries and replies.