Sustainable Nitrogen Fertilizer: Solar thermochemical production of ammonia from water, air and sunlight, thermodynamic and economic analyses
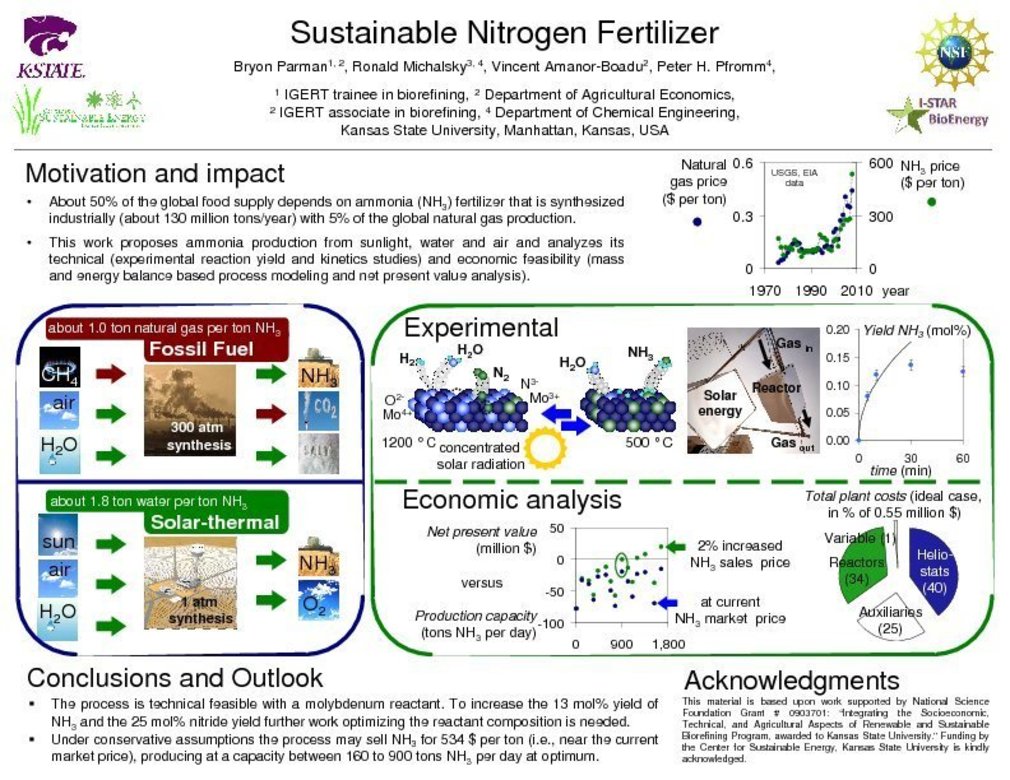
Ammonia is an important input into agriculture and is used widely as base chemical for the chemical industry. It has recently been proposed as a sustainable transportation fuel and convenient one-way hydrogen carrier. Employing typical meteorological data for Palmdale, CA, solar energy is considered here as an inexpensive and renewable energy alternative in the synthesis of NH3 (Anhydrous Ammonia) at ambient pressure and without natural gas. Thermodynamic process analysis shows that a molybdenum-based solar thermochemical NH3 production cycle, conducted at or below 1500 K, combined with solar thermochemical H2 production from water may operate at a net-efficiency ranging from 23-30% (lower heating value of NH3 relative to the total energy input). Net present value optimization indicates ecologically and economically sustainable NH3 synthesis at above about 160 tons NH3 per day, dependent primarily on heliostat costs (varied between 90 and 164 dollars/m2), NH3 yields (ranging from 13.9 mol% to stoichiometric conversion of fixed and reduced nitrogen to NH3), and the NH3 sales price. Economically feasible production at an optimum plant capacity near 900 tons NH3 per day is shown at relative conservative technical assumptions and at a reasonable NH3 sales price of about 534 ± 28 dollars per ton NH3.



Judges and Presenters may log in to read queries and replies.